Liebscher Lab
Advancing Disease Neurobiology Through Circuit Mechanisms
Sabine Liebscher is a neurobiologist and medical doctor whose research focuses on unraveling the cellular and circuit mechanisms underlying CNS disorders, with the ultimate goal of identifying new therapeutic strategies. As the current Professor for Systemic Neurobiology at the Medical University of Innsbruck and formerly at the University of Cologne and LMU Munich, she bridges clinical insights with cutting-edge basic research to better understand diseases such as amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, and autoimmune encephalitis.
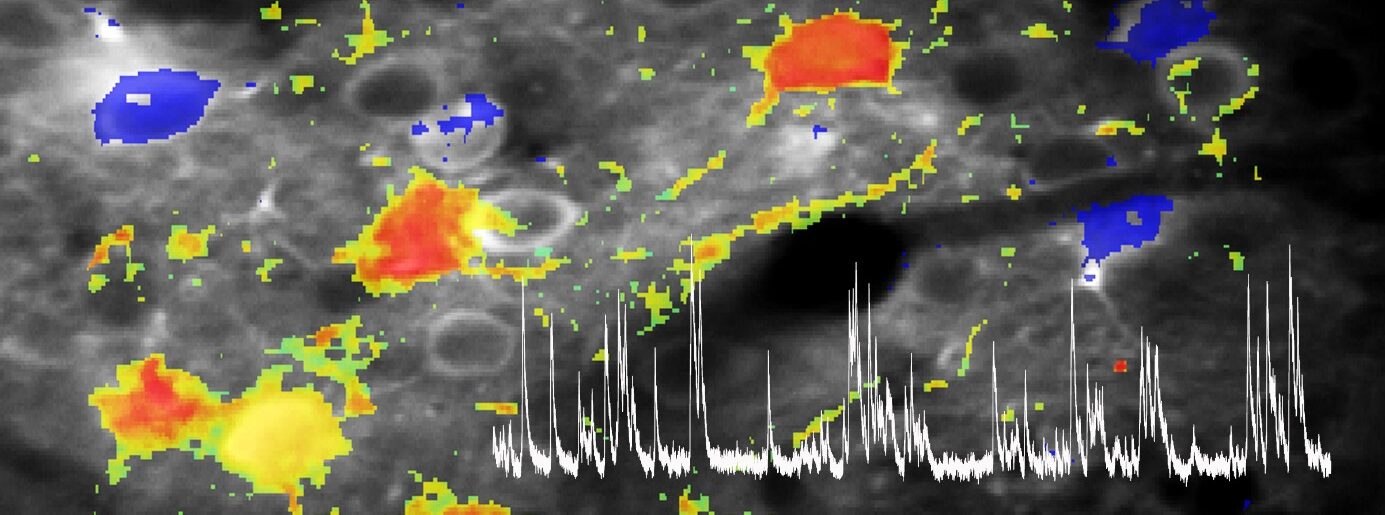
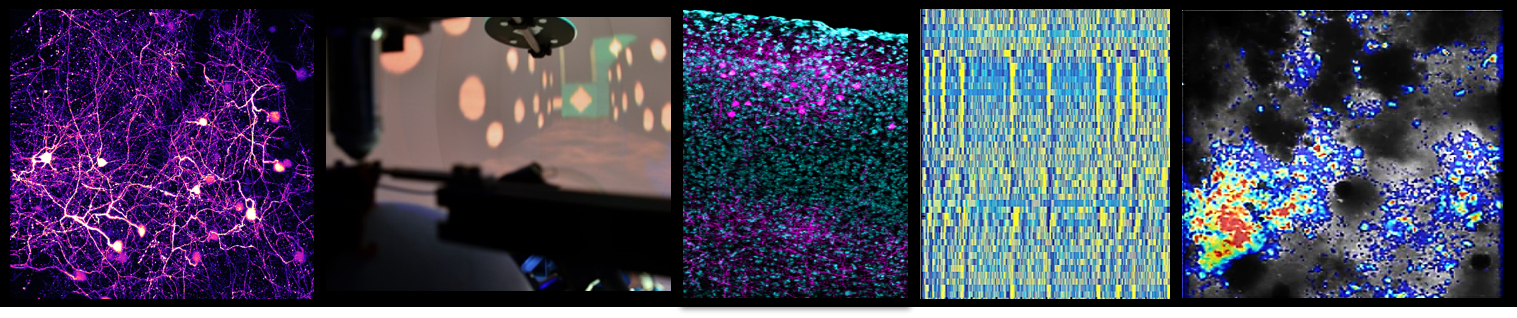
Her lab employs state-of-the-art in vivo imaging techniques, particularly two-photon microscopy, to chronically observe the structure and function of neurons and glial cells at single-cell and synapse resolution in behaving animal models. This approach is complemented by chemogenetic manipulation, single-cell transcriptomics, proteomics, and circuit mapping, enabling the team to dissect how molecular and cellular changes translate into network dysfunction underlying typical clinical symptoms. A central goal the translation of findings from animal models to human disease, is achieved through close collaboration with clinicians and neuropathologists, to foster the development of novel diagnostic and therapeutic approaches, including gene therapies and targeted interventions. Her work continues to illuminate how disruptions in neural circuits drive neurodegeneration, paving the way for more precise and effective treatments for CNS disorder.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a devastating, progressive neurodegenerative disorder that primarily affects the nerve cells in the brain and spinal cord responsible for controlling voluntary muscle movement. As motor neurons degenerate and die, individuals experience increasing muscle weakness, loss of mobility, difficulty speaking and swallowing, and ultimately respiratory failure. Despite its severity and fatal outcome, with most patients surviving only three to five years after symptom onset, the exact causes of ALS remain unclear and there is currently no cure. Further research is critical to unravel the complex mechanisms driving ALS, identify early biomarkers, and develop effective therapies that can slow or halt disease progression, offering hope for improved outcomes and quality of life for those affected.
A central question in the field is how well-known neuropathological features, such as cytoplasmic protein aggregates or the nuclear depletion of those proteins, affect neuronal and network function and why these alterations, despite not being selectively observed in motor neurons, would however primarily drive motor neuron death.
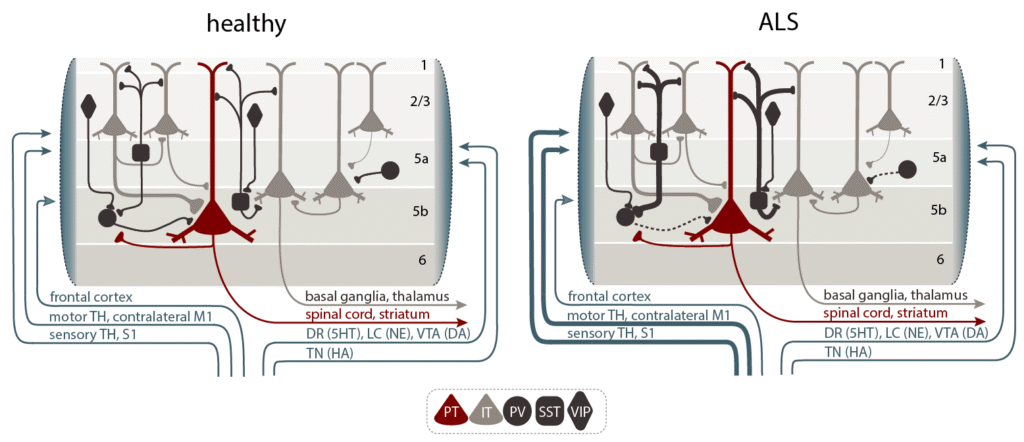
Our research on ALS thus focuses on unraveling how disruptions in neural circuits and glial function drive the disease process. ALS is not solely a disease of motor neuron degeneration, but involves complex alterations in the structure, function, and connectivity of entire motor circuits, including neuromodulatory systems, in both the cortex and spinal cord. Using advanced in vivo imaging techniques, we investigate how early features, like cortical hyperexcitability, emerge and contribute to the progression of ALS. This circuit dysfunction is seen as a key factor in both symptom development and the spread of neurodegeneration.
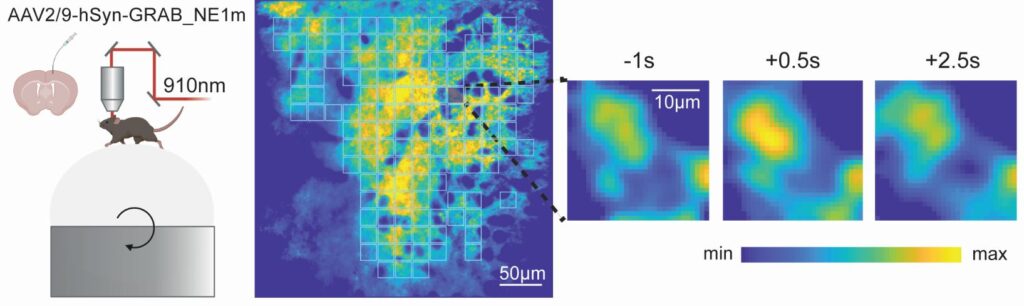
We are also placing a strong emphasis on the role of astrocytes, the most abundant glial cells in the brain. By combining in vivo calcium imaging with transcriptomic and proteomic analyses, we characterize how astrocyte signaling becomes altered in ALS and identify molecular candidates that may drive this dysfunction. Genetic rescue experiments are then used to test whether restoring healthy astrocyte function can protect motor neurons and improve circuit health.
We strive to provide a more comprehensive understanding of ALS by integrating the role of individual circuit elements including glial cells and their interactions with neural circuits, highlighting multifaceted, cell type- and stage-specific disturbances in excitation/inhibition balance as a cardinal aspect of ALS pathophysiology.
Autoimmune encephalitis
Autoimmune encephalitis is a severe neurological disorder in which the immune system mistakenly targets components of the central nervous system, often leading to a wide range of psychiatric and neurological symptoms. One of the most common forms, anti-NMDA receptor (NMDAR) encephalitis, is caused by autoantibodies against NMDA receptors, resulting in symptoms that progress from psychiatric disturbances (such as psychosis) to seizures, movement disorders, and cognitive deficits. While early treatment can lead to recovery, many patients suffer from persistent cognitive or affective impairments.
Our research focuses on uncovering the cellular and circuit-level mechanisms underlying autoimmune encephalitis caused by various autoantibodies, directed e.g. against the NMDAR or IgLON5. We use advanced in vivo two-photon imaging in behaving mouse models, combined with genetically encoded indicators and passive infusion of patient-derived autoantibodies, to chronically monitor how specific neurons and glial cells are affected during disease progression. By mapping structural and functional deficits in different neuronal subtypes and circuits, we aim to understand how autoantibodies disrupt network function and lead to the broad spectrum of symptoms seen in patients.
Our approach integrates circuit mapping, chemogenetic manipulation, and transcriptomic/proteomic analyses to identify which neuronal populations and pathways are most vulnerable, and to validate findings in human samples. This research not only provides new insights into the pathophysiology of autoimmune encephalitis but also seeks to identify potential therapeutic targets to improve long-term outcomes for patients.