Research
I. Development of an Open Tripartite Microfluidic Chamber for Comparative Axon Outgrowth Studies
Cultures of primary mouse neurons are a cornerstone of in vitro axon outgrowth studies. These neurons can be obtained from various mutant mouse strains and exposed to different experimental conditions once cultured. Axon outgrowth is typically assessed by imaging individual neurons and semi-automatically quantifying axonal length—a tedious process, particularly when analyzing large cohorts in low-density cultures.
To accelerate and standardize this process, we adopted microfluidic devices designed to fluidically separate neuronal cell bodies from their axons. Traditional chamber designs feature enclosed somatic and axonal compartments, with neurons seeded via top and bottom wells. However, we found that this closed architecture places stress on neurons and hampers axonal growth—likely due to inadequate nutrient supply and poor waste removal.
To address these limitations, we collaborated with Xona Microfluidics, a leading U.S.-based manufacturer of microfluidic devices. Together, we developed a prototype of a novel tripartite open-compartment chamber. This new design features two open lateral somatic compartments for direct neuron seeding and a wide, closed central compartment for axon growth.
This architecture significantly simplifies neuron seeding and enables direct comparison of axon growth from two distinct neuronal populations—such as wild-type and knockout neurons—within the same device. Notably, axon growth from several hundred neurons can be measured simultaneously, greatly enhancing the statistical power of our experiments. We also developed custom MATLAB scripts for automated quantification of axon growth within the central compartment.
This chamber type is now commercially available from Xona Microfluidics under the name “Innsbruck Device”. We currently use it to characterize axonal growth in various knockout and knock-in mouse strains.
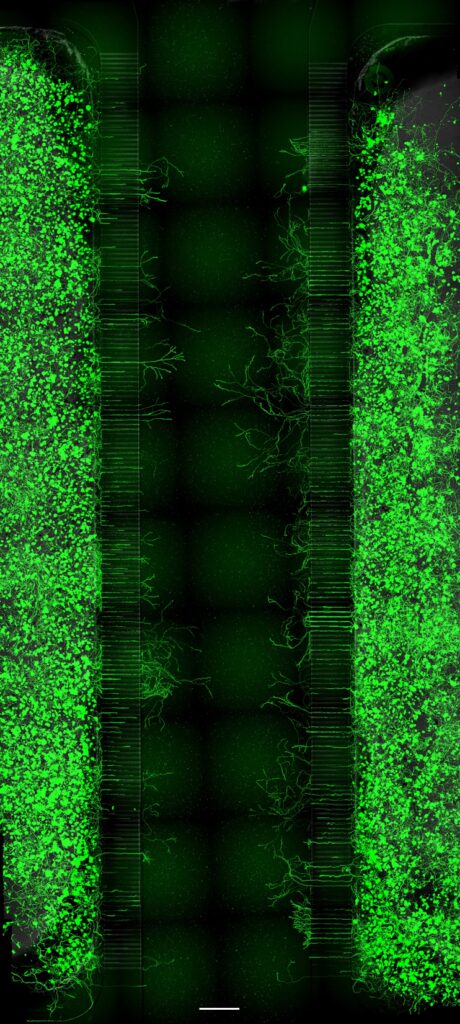
DRG neurons from adult mice were seeded into the open lateral compartments, and NGF was added to the closed central compartment to chemoattract axons through the microgrooves. Cultures were fixed 42 hours post-seeding and stained with Tuj1. Axon growth from both neuronal populations can be quantified and compared. Scale bar = 500 µm.
II. Development of a Clinically Relevant Experimental Peripheral Nerve Injury Model in the Mouse
Unlike axons in the central nervous system (CNS), peripheral nerves are widely believed to regenerate spontaneously without significant clinical intervention. However, traumatic injuries involving nerve transection do not regenerate on their own. These severe injuries require surgical intervention, and even then, functional outcomes are often poor, resulting in lifelong sensory and motor deficits.
Autologous nerve transplantation remains the gold standard for surgical repair. However, advances in biocompatible materials are driving the clinical adoption of synthetic nerve conduits. Despite these developments, virtually all experimental peripheral nerve injury (PNI) studies rely on models with limited clinical relevance, such as crush lesions or direct stump ligation. These approaches do not yield clinically meaningful data and do not permit testing of molecular compounds at the lesion site.
To overcome these limitations, we have developed a clinically relevant mouse model of peripheral nerve injury that closely mimics human clinical conditions and allows clinically relevant conclusions to be drawn. The key features of our model include:
- Transection-Based Lesion: The sciatic nerve is transected and the stumps are sutured to a chitosan conduit. This procedure involves both surgical intervention and reconstruction. While technically more demanding than comparable rat surgeries, mice offer access to numerous genetically modified strains unavailable in rats. Chitosan conduits are increasingly used in clinical settings due to their excellent biocompatibility.
- Localized Application of Molecular Compounds: Soluble molecular compounds can be applied directly to the lesion site before the conduit is sealed, enabling evaluation of their potential to enhance axonal regeneration and sensory-motor recovery.
- Single-Axon Visualization and Quantification: We developed a method to visualize and quantify regenerating nerve fibers at single-axon resolution within the conduit, providing high-resolution morphological data.
- Functional Recovery Assessment: After a recovery period, operated mice undergo a series of behavioral tests—including ladder walk, Von Frey, and CatWalk assays—to evaluate functional improvement.
We currently use this novel lesion model to identify and characterize soluble molecular compounds that promote axonal growth and functional recovery, with the long-term goal of evaluating their therapeutic potential in clinical trials.
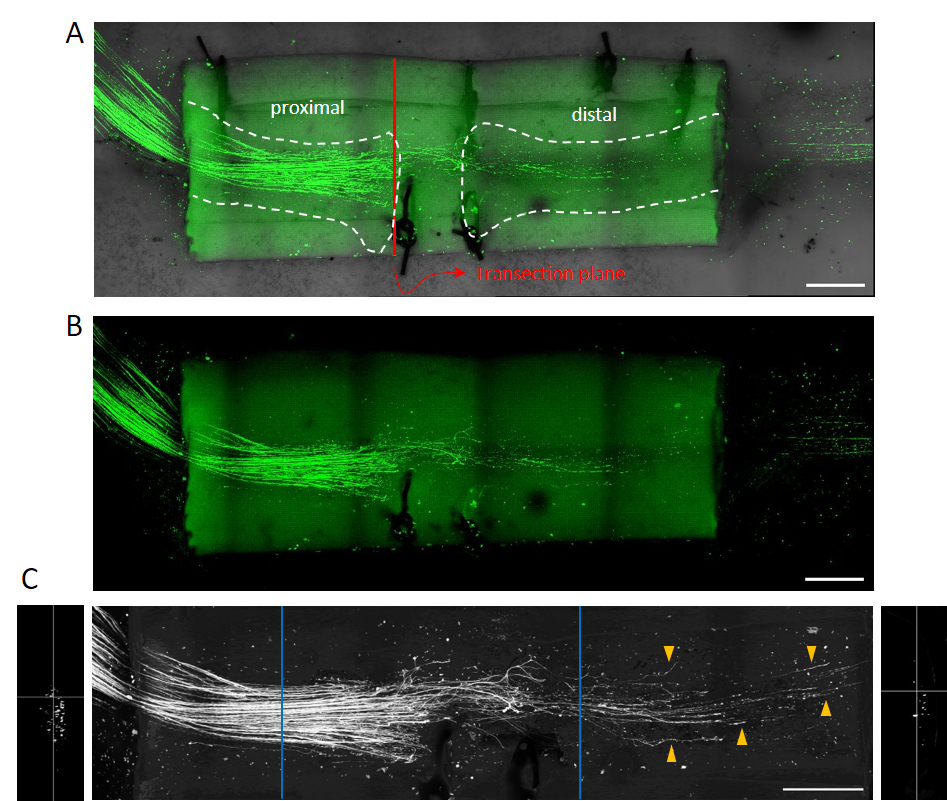
Following transection of the sciatic nerve—the largest peripheral nerve in the body—the stumps are sutured to a chitosan conduit. Soluble molecular compounds, hypothesized to enhance regeneration, can be applied directly at the lesion site. After 14 days, the conduit is removed and subjected to confocal imaging.
(A) Combined transmission light and EGFP fluorescence image. A GFP-M mouse, which expresses EGFP in ~1% of axons, is used. While distal axons undergo Wallerian degeneration, the overall nerve structure remains intact. Dashed lines indicate nerve stumps within the conduit.
(B) EGFP fluorescence only.
(C) EGFP signal is processed by AI to subtract background and conduit autofluorescence and isolate axonal signals. Regenerating axons in the distal stump are marked by orange arrowheads, illustrating that only a small fraction of axons initiate regeneration—one reason for poor functional recovery. Orthogonal slices of proximal and distal stumps are shown with respective positions marked in blue. EGFP-positive intersections are quantified along the conduit axis in 1 µm increments. Scale bar = 500 µm.